The role of protein degradation during infection
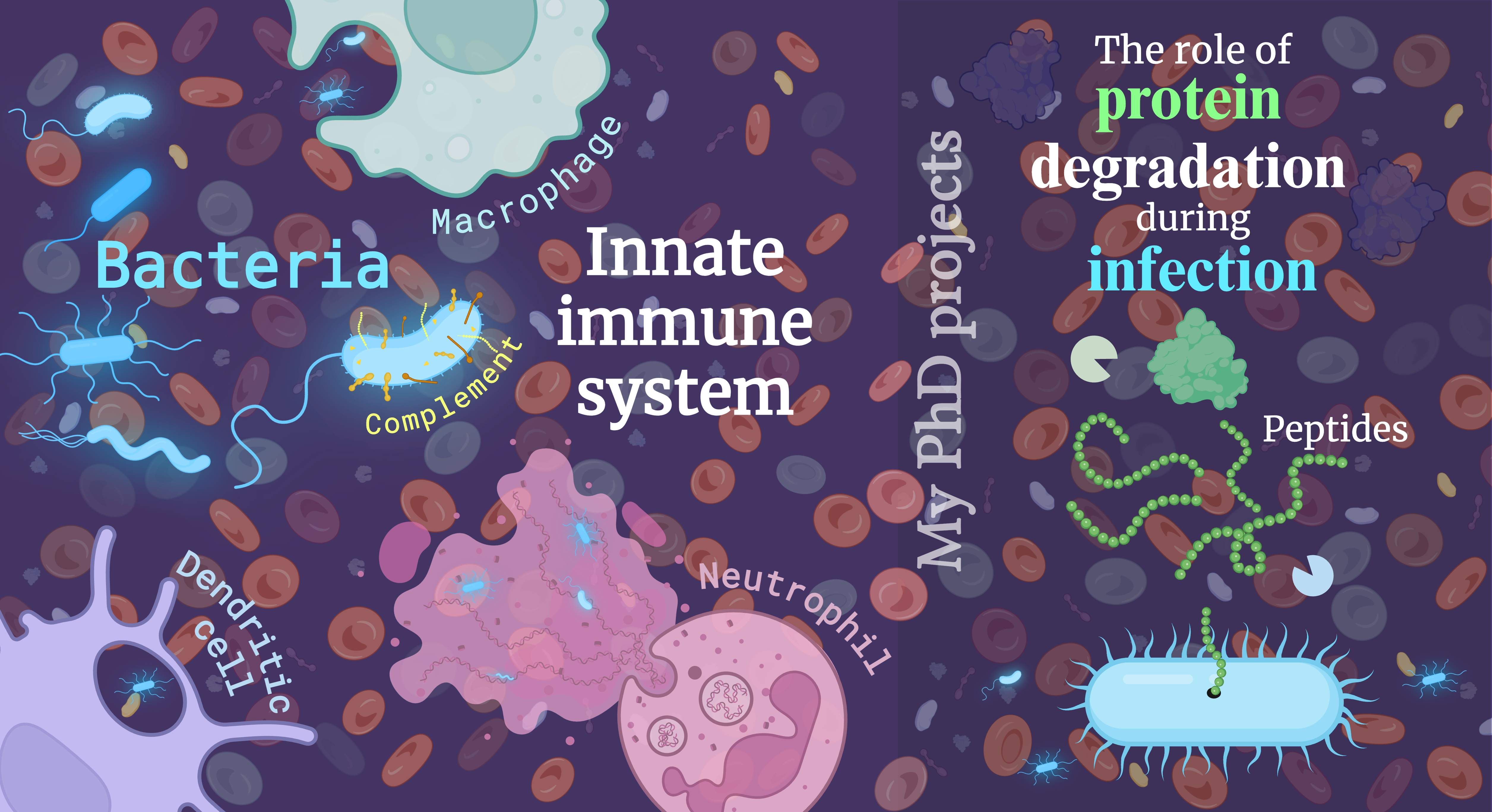
The immune system is one of the most complex systems in living organisms (seconded only by the brain, in my opinion). It constantly fights the never-ending invasion of bacteria, viruses and other pathogens in order to keep you alive. Most of the time, it does so without you even noticing. Although there is a huge research field dedicated to understanding the immune system (called immunology) many of its complexities are yet to be discovered and understood. Having recently started my journey towards a PhD as a computational biologist with the goal to understand one such hypothetical mechanism, I figured I’d write a short piece about the reasoning and significance of my research - both for myself, and for anyone who’s curious.
The immune system is a complex network of cells and molecules that work together to identify and kill pathogens. It is generally divided into two main subsystems: the innate immune system and the adaptive (or acquired) immune system. The innate immune system acts as the body’s first line of defense, providing an immediate but generalized response to threats. It is also the focus of my work. In contrast, the adaptive immune system is more specialized, taking longer to respond but doing so with precision and memory that allows for a more effective defense against specific pathogens.
Of the two systems, the innate immune system is comparatively ancient, originating over a billion years ago when life was in its infancy. This long evolutionary history establishes two things: the innate immune system is both very important and by now it should be effective. Over this immense time span, natural selection has honed the innate immune system into a highly effective, albeit broad, defense mechanism - tying up loose ends and streamlining it to near perfection.
We can think of the innate immune system as an army, with various cells and molecules playing specialized roles. For example, macrophages, the “big eaters,” serve as the frontline soldiers, rapidly engulfing and destroying invaders. Dendritic cells act as intelligence officers, gathering critical information about the enemy to inform the adaptive immune system. Not all of these “soldiers” are cells; some are molecules like the proteins of the complement system, which swarm and attack pathogens, either directly killing them or making them easier to hunt down by other immune cells. This well-orchestrated defense system may seem engineered with precision, but in reality it is the result of hundreds of millions of years of blind evolutionary refinement. Amazing.
In my PhD research, I aim to explore what might be a new category of “soldiers” within this innate army. Unlike traditional soldiers, these are not physical entities but mechanisms — more like weapons or tactics.
To understand the concept, we have to establish some basics. Biological systems are almost exclusively built from amino acids, nucleotides, fats, and sugars. Chains of amino acids make up proteins. These are especially vital, performing a vast array of functions necessary for the structure, function, and regulation of cells, tissues, and organs. Proteins catalyze biochemical reactions, act as messengers and receivers, regulate molecular and cellular movement, and provide structural integrity to cells. Importantly, they play an essential role in the immune system. The proteome, or the complete set of proteins within a cell, is dynamic, constantly adapting to the environment and the needs of the organism. Thus, it is crucial that proteins no longer needed are degraded and recycled, allowing the cell to reclaim valuable resources while adjusting its functions to meet current demands.
When proteins are degraded, they are broken down into smaller fragments called peptides. Recent research has revealed that these peptides are not just by-products of degradation; many have critical functions of their own. For instance, some peptides exhibit antimicrobial properties, disrupting the membranes of bacteria and making their stay in the body rather unpleasant, and may even come to an immediate termination. Others can bind to receptors, influencing cell signaling pathways. This raises a compelling question: could evolution have shaped a system where protein degradation itself serves a dual purpose—recycling materials while simultaneously generating functional peptides that aid in the immune response? If so, protein degradation might be an active component of the innate immune system, providing another mechanism in the battle against pathogens.
From the perspective of pathogens, many of the host’s proteins are very dangerous, sometimes even lethal (like the complement system!). Consequently, degrading these proteins could be a strategic move for a pathogen, dismantling the host’s defenses and facilitating infection. This interplay between host and pathogen, where each tries to outmaneuver the other by degrading proteins, adds another layer of complexity to the immune response.
Understanding protein degradation and peptide generation in this context could reveal significant insights into the innate immune system and the ongoing battle between pathogens and hosts. My PhD research focuses on investigating whether this mechanism indeed plays a vital role in immunity. Preliminary evidence suggests that protein degradation patterns indeed do vary during infections, hinting at a deeper involvement of this process during infection. To explore this further, I am developing mathematical and computational models that aims to provide a comprehensive understanding of how protein degradation occurs and how it differs under various conditions, potentially unveiling new diagnostic tools, therapeutic targets or strategies to bolster the immune response.
Enjoy Reading This Article?
Here are some more articles you might like to read next: